UCSF Innovation
The Living Therapeutics Initiative (LTI) provides support to UCSF cellular therapy projects across a broad range of diseases, including solid and hematological cancers, autoimmune diseases, immune deficiencies, neuro-inflammatory diseases, and others. In addition to advancing high risk innovation that industry rarely undertakes, the LTI also helps address the “valley of death” in the development process between pre-clinical validation and testing new therapies in clinical trials. Generating cellular therapies that can be administered clinically requires that they are made with current Good Manufacturing Practices (cGMP) to ensure that the products are consistently produced to meet FDA quality standards. Converting a product made at research scale to cGMP manufacturing typically takes more than two years to complete, is expensive, and grant funding is limited.
To address bottlenecks in cGMP manufacturing, UCSF established a partnership with Thermo Fisher Scientific for additional capabilities supporting the advancement of UCSF cell therapy innovations into and through clinical testing. As a result, manufacturing of LTI projects is supported both internally through the UCSF Investigational Cell Therapy Program and by Thermo Fisher Scientific, whose manufacturing facility is located on the UCSF campus in the Nancy and Stephen Grand Building. These programs support early and late-stage process development, preclinical studies, regulatory submissions, and clinical cGMP manufacturing. The partnership with Thermo Fisher Scientific additionally provides access to commercial-grade cGMP manufacturing services.
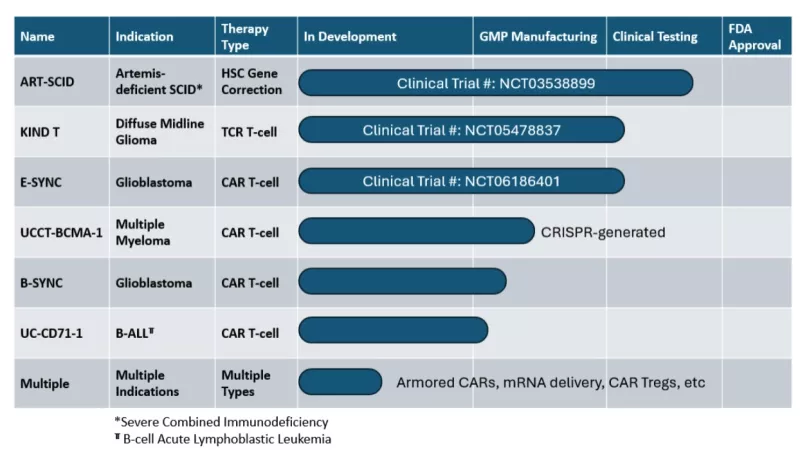
Within the LTI portfolio of associated projects, there are three therapies currently in clinical testing, three therapies in cGMP manufacturing, and numerous projects in preclinical development (see table).
Clinical trial links:
Cell Therapies and Ultra-Rare Diseases
Cellular therapies offer the promise of treatments, and possibly cures, for a range of genetic diseases, particularly within hematopoietic (blood) cells. However, many of these diseases are ultra-rare, meaning that fewer than 1 in 50,000 people are affected. At these low patient numbers, there are effectively no industry partners interested in out-licensing and developing these therapies commercially.

Furthermore, developing therapies for ultra-rare diseases is hampered by the current regulatory frameworks which are not designed to address the challenges associated with approving drugs for such small patient populations. The FDA and global regulatory agencies recognize that this is an issue and are slowly making inroads to address the problems. Working with academic organizations to find long-term solutions for maintaining access has the potential to keep these treatments available to patients.
Without the potential of commercial partners, there are challenges in bringing our highly-effective therapeutics for ultra-rare diseases into standard of care once clinical testing is completed. UCSF faculty, Mort Cowan, MD, and Jennifer Puck, MD, have developed a highly effective, paradigm changing hematopoietic stem cell (HSC) therapy for babies born with Artemis-deficient, severe-combined immunodeficiency (ART-SCID), an ultra-rare condition (fewer than five US/Canadian patients born per year) in which babies are born without a functioning adaptive immune system. These babies lack T cells and B cells which are essential for not only fighting bacterial, viral and fungal infections but also for surveillance against emerging cancers.
Babies with ART-SCID, unfortunately, are not good candidates for the standard of care therapy, a bone marrow transplant (BMT) using a healthy donor’s (allogeneic) bone marrow hematopoietic stem cells. The Artemis protein, an essential component of DNA repair, is non-functional in these babies, making them significantly more susceptible to ionizing radiation and alkylating chemotherapy, commonly used to prepare patients for an allogeneic BMT. These babies also have an increased risk BMT rejection and are more likely to experience graft-vs-host disease (GVHD), a reaction that occurs when donor T cells react against tissue antigens in the recipient. Dr. Cowan’s and Puck’s ART-SCID cellular gene therapy corrects the gene defect in the patients’ own blood forming hematopoietic stem cells, eliminating the need for an allogeneic BMT and consequently eliminating rejection, GVHD and long-term consequences from high-dose chemotherapy.
Artemis-deficient SCID, if left untreated, is fatal in infancy. Prior to the ART-SCID cellular therapy, the survival rate among affected babies using standard of care treatment (allogeneic BMT) was around 60% and in 90% of the surviving children the immune system did not fully reconstitute unless high dose chemotherapy was administered. However, the patients receiving high-dose alkylating chemotherapy had poor survival and if they did survive, were subjected to significant late effects and poor quality of life. By comparison, to date in the ART-SCID cellular gene therapy clinical trial, the survival rate has been 100% with >85% of the treated children achieving complete or near complete immune reconstitution. While it is still too early to tell, the ART-SCID cellular gene therapy has the promise of curing these young patients. UCSF is working to keep this therapy available for those who need it.
Patient Resources
The following links provide information about the cellular therapy clinical practice at UCSF