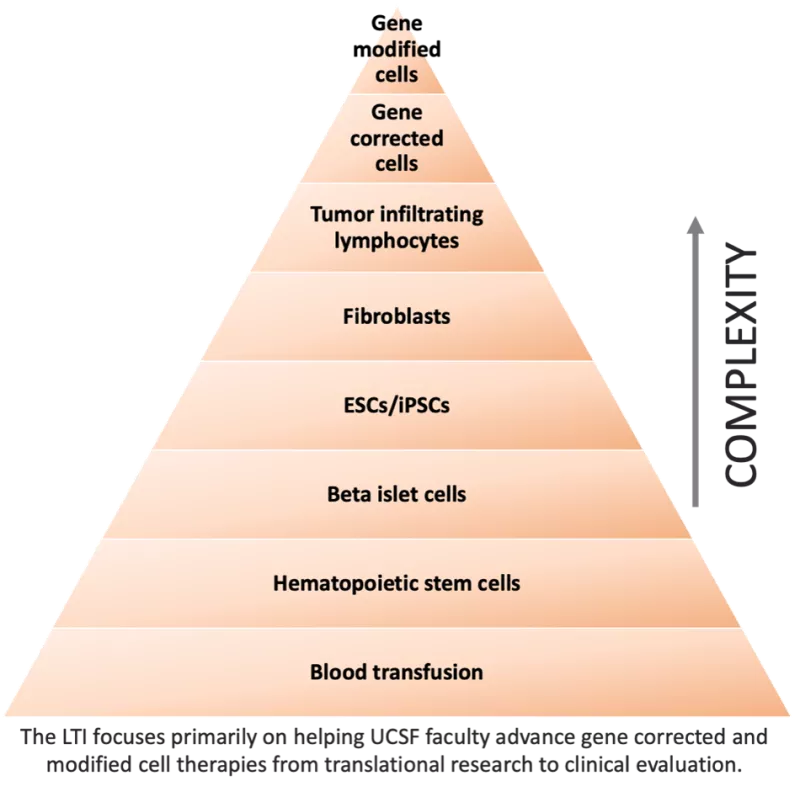
Cellular therapy uses a patient’s own- and sometimes another person’s cells- to eradicate or correct a wide range of disorders. As a therapeutic modality, cell therapies are not new. Physicians have been performing blood transfusions and hematopoietic stem cell transplants for decades. What makes the current wave of cellular therapies so exciting is that now scientists are able to genetically modify cells to either correct disease-causing gene defects or turn them into powerful weapons for treating disease. The cellular therapy pyramid (pictured below) provides a summary of the range of cellular therapies.
Cellular therapies at the top of the pyramid burst into public view in 2017 with the approval of Kymriah, a CD19 Chimeric Antigen Receptor (CAR) T-cell for the treatment of B-cell acute lymphoblastic leukemia in children and adults. There are now seven CAR T-cell therapy approvals for B-cell based cancers. Developing cellular therapies for solid tumors has proven more challenging. However, in 2024, a tumor infiltration lymphocyte (TIL) therapy, Amtagvi, was approved for metastatic melanoma and a T-cell receptor (TCR) T-cell therapy, Tecelra, was approved for synovial sarcoma.
Exciting, too, are the successes in correcting gene defects in hematopoietic stem cells. While still too early to know their long-term efficacy, it is hoped that correcting the defect at the hematopoietic stem cell level will lead to cures. In 2023, the FDA approved two gene-modified hematopoietic stems cell therapies for sickle cell disease (SCD), Casgevy and Lyfgenia. In 2024, they approved Lenmeldy, which corrects the genetic defect in hematopoietic stem cells to treat metachromatic leukodystrophy, a rare genetic disease affecting the brain and nervous system.
Cell therapy to improve patient outcomes: